Introduction
Anyone familiar with metals can readily recognize the differences between brass and bronze, but why are we singled out for discussion? You’ve probably also noticed that when choosing between brass and bronze, you hesitate. This is because you lack a detailed understanding of the pros and cons of each. Their composition, performance, and applications are vastly different. If you share these concerns or questions, please read the following article with your questions in mind. We hope the following descriptions offer new insights or valuable insights for you.
How They Are Made
Brass and bronze are alloys of copper, and their primary composition is copper. Why is copper alloy (bronze and brass) necessary? Because copper itself has certain drawbacks, copper alloys incorporating other metals can avoid these drawbacks of pure copper and make the new alloys more suitable for certain applications. Whether brass or bronze, they all undergo the same four steps: smelting, alloying, casting, and processing. We’ll describe how copper alloys are produced below:
1. The First Step Is Smelting
Smelting involves melting copper and the desired metal in a high-temperature furnace according to the desired ratio. Temperature control is crucial. If the temperature doesn’t reach copper’s melting point, it won’t melt. However, excessively high temperatures can cause some of the metal to evaporate, ultimately resulting in an unsatisfactory alloy or wasted smelting energy.
2. The Second Step Is Alloying
Alloying means adjusting the ratio of copper to other metals to ensure the correct proportions in the final furnace. Other alloying elements may also be added to improve properties as needed. For example, although brass is an alloy of copper and zinc, small amounts of tin, lead, iron, or manganese are sometimes added to enhance its hardness and machinability.
3. The Third Step Is Casting
Casting involves pouring the copper alloy from the furnace into a designated mold according to the application requirements, or directly forging it into plates, bars, or tubes.
4. The Final Step Is Heat Treatment and Processing
Depending on the metal’s intended use, processes such as annealing, cold working, stretching, and rolling are performed to achieve the desired hardness and toughness. Heat treatment, which means annealing, overlaps with other processing steps because copper may harden during processing (cold work hardening), potentially causing deformation or fracture. Annealing is like a “relaxing massage” and “resetting” process for the metal, allowing it to recover from the fatigue of cold, hard processing and become “soft and malleable” again, or at least “relaxed and stress-free,” preparing it for subsequent processing or safe use.
Brass vs. Bronze: Key Differences at a Glance
“Even someone who knows nothing about metals can intuitively distinguish between two common types of ‘copper’: brass, which gleams golden and resembles 18K gold; bronze, which has a darker, bluish-gray brown hue. This most intuitive color difference is just the tip of the iceberg of their vast differences. Hidden behind these different hues are completely different ‘recipes’ and the unique properties that match them. We understand that you need a more comprehensive explanation, not only of the differences that can be seen, but also of the differences that cannot be seen with the naked eye. Below we will explain the differences between brass and bronze from the basics to the advanced level:
Composition
Brass
Brass contains mostly copper, zinc, and small amounts of other trace elements .The property (level of strength, hardness, resistance to corrosion, and ability to be machined) of brass is primarily changed by the zinc content and other alloying additions.
The most simple brass is a binary alloy consisting simply of copper and zinc.The main reason for adding zinc is to reduce costs and improve the metallurgy. properties of brass a higher zinc content reduces expense, but also initial rectitude and plasticity increases, and then decreases again. At the cost of certain properties,however, a third,or more elements may be added to copper and zinc.
Lead (Pb): Lead is, in the space of copper,insoluble but may exist as tiny particles in copper alloy. Its addition significantly improves the machinability of brass, makes for easier turning and milling, and so is suitable for making precision parts like valves and watch components.
Tin (Sn): When added to copper it allows for the creation of tin brass, such as HSn90-1 (also known as naval brass). This primary use of tin in brass is to improve resistance to seawater corrosion, so it is widely used in ship parts.
Aluminum (Al),Manganese (Mn),Iron (Fe): These are primarily used to increase the strength, hardness and wear resistance of brass.
Bronze
Bronze originally referred specifically to tin bronze, a copper-tin alloy. Traditional bronze provides excellent wear and corrosion resistance, a low casting shrinkage rate (resulting in accurate mold reproduction), and is only slightly less fluid when cast. It is frequently used for springs, wear-resistant parts of any kind, and artistic castings.
But the modern definition of “bronze” is broader, including copper-based alloys with other primary alloying elements instead of zinc and nickel (note: white copper is a copper-nickel alloy, and brass is a copper-zinc alloy).
Aluminium bronze: Aluminium (Al) is the principal alloying element (Al is usually in the range of 5-12% Al). It has good strength, excellent wear resistance and good resistance to atmospheric and seawater corrosion, and so is known as ‘golden steel’. Its price is generally lower than that of tin bronze.
Phosphor bronze: A small amount of phosphorus (P) is added to tin bronze. Phosphorus serves as a deoxidizer, and increases an alloy’s elasticity and wear resistance. Ideal for elastic components like reeds, diaphragms and electrical sockets.
Silicon bronze: Aluminium (Si) is the principal alloying element. Stand-up castability, excellent weldability and corrosion resistance, good mechanical performance. Serves frequently as a replacement for tin bronze, in order to reduce costs.
Color/Appearance (Why It Is That Way)
The most intuitive way to distinguish brass from bronze is by observing their color. This is also the quickest and most convenient way to tell the difference. You’ll notice that, because brass contains a high zinc content, it has a golden, shimmering color, resembling “fake gold.” Meanwhile, bronze, due to its inclusion of tin (or aluminum, phosphorus, etc.), has a more bluish-gray/dark brown color.
It’s worth noting that brass readily forms zinc oxide (ZnO) and copper oxide (Cu₂O, CuO) on its surface due to oxidation. Zinc oxide is white, while copper oxide is reddish or black. The combination of these two oxides still maintains a relatively bright golden hue. However, bronze, when oxidized, primarily forms tin oxide (SnO₂, off-white) and copper oxide on its surface. The combination of these oxides creates a dull, bluish-gray appearance.
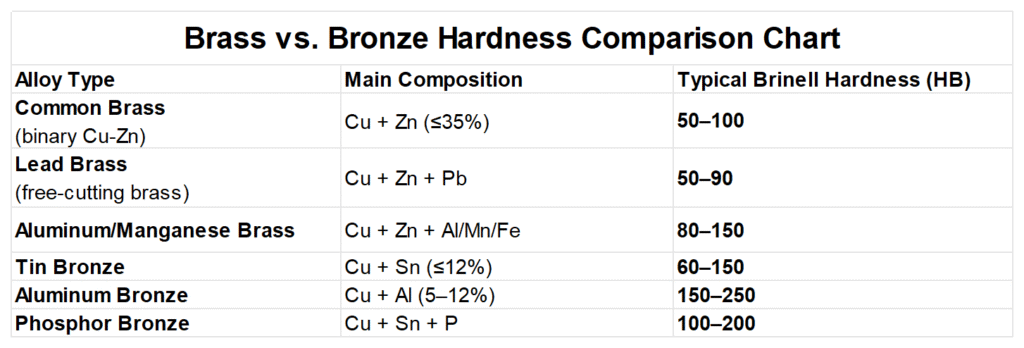
Strength and Hardness
As you can see from the above description, there’s no absolute uniform hardness difference between brass and bronze. Their hardness depends on the alloying elements and heat treatment methods. However, there are some consistent patterns, please check the chart below:
Corrosion Resistance
When it comes to corrosion resistance, you often hear that bronze is more resistant than brass. However, the truth is that brass also has excellent corrosion resistance, but only in atmospheric or freshwater environments. Brass’s corrosion resistance deteriorates in seawater because, in chloride-containing environments (seawater, salt spray), the zinc in brass dissolves when exposed to chloride ions, leaving behind a porous copper matrix (dezincification). This form of corrosion significantly reduces its strength.
Bronze, on the other hand, is different. Whether it’s traditional tin bronze or modern aluminum bronze and phosphor bronze, the addition of other metals enhances copper’s resistance to seawater corrosion. So many of the copper parts you see used in the marine field are made of bronze. This is why bronze is generally considered more corrosion-resistant than brass.
Machinability
Although both are copper alloys, the differences in processing properties between brass and bronze are quite significant due to the unique properties of other metal elements. You may also want to know what processing methods are suitable for each. So let’s discuss the differences between bronze and brass from four processing dimensions:
1.Machinability
Brass, due to its zinc content, is harder and more brittle than pure copper. Furthermore, the presence of a small amount of lead provides lubrication and chip breaking, resulting in excellent cutting performance。Therefore, brass can be cut using the commonly used CNC cutting method, which causes less wear on the tool and has high processing efficiency.
Bronze, on the other hand, is different. Its alloying elements (such as tin and aluminum) primarily enhance hardness and wear resistance, but do not improve machinability. Instead, they make cutting more difficult and result in greater tool wear during machining. Therefore, it is best suited to forming through casting or heat treatment, rather than relying on high-speed cutting.
2. Formability / Ductility
Brass has excellent plasticity and is primarily used in the manufacture of sheet, strip, and tube. Furthermore, because brass easily undergoes cold working (cold stamping, drawing, bending, etc.), it is widely used in decorative components for electronic devices and even in essential public services such as heat sinks. Bronze, on the other hand, has low ductility and poor cold workability, and can usually only be formed into various materials through hot forming (forging, extrusion) or casting.
3. Weldability & Brazing
In practice, many bronzes—especially tin and phosphor bronze—tend to weld more easily than brass. That’s why they are commonly chosen for repair work or adding wear-resistant layers. It’s worth noting that the weldability of different bronze types varies significantly. For example, aluminum bronze tends to form a dense oxide film during welding, requiring special protective measures.
By contrast, brass is less weldable due to the high-temperature volatilization of zinc during welding, which can easily cause weld porosity and embrittlement. However, using silver-based brazing filler metals can still achieve a strong and reliable connection with brass.
4. Castability
Both brass and bronze possess excellent forgeability, but their forging applications are suited to different applications. Brass is suitable for stamping, cold extrusion, and turning, making it more suitable for forging into small, high-precision parts (such as screws, plugs, valve cores, and electronic components).
Bronze is primarily an alloy of tin, aluminum, silicon, and phosphorus. These elements give it high strength, wear resistance, and fatigue resistance, making it more suitable for hot forging or casting into large parts (such as bearing seats, gears, ship parts, and sculptures).
Cost
Here, you’ll see that the costs of brass and bronze are primarily influenced by two factors: raw material and processing costs.Traditional tin bronze is more expensive because tin is much more expensive than zinc (zinc primarily affects brass costs). Aluminum bronze and silicon bronze, while not as expensive as tin, are still more expensive than brass overall. Furthermore, bronze’s processing costs are higher (it’s difficult to cut and relies more on casting/forging), so overall, bronze costs more.
Which Metal Should You Choose for Your Project?
Recommendations by Common Application:
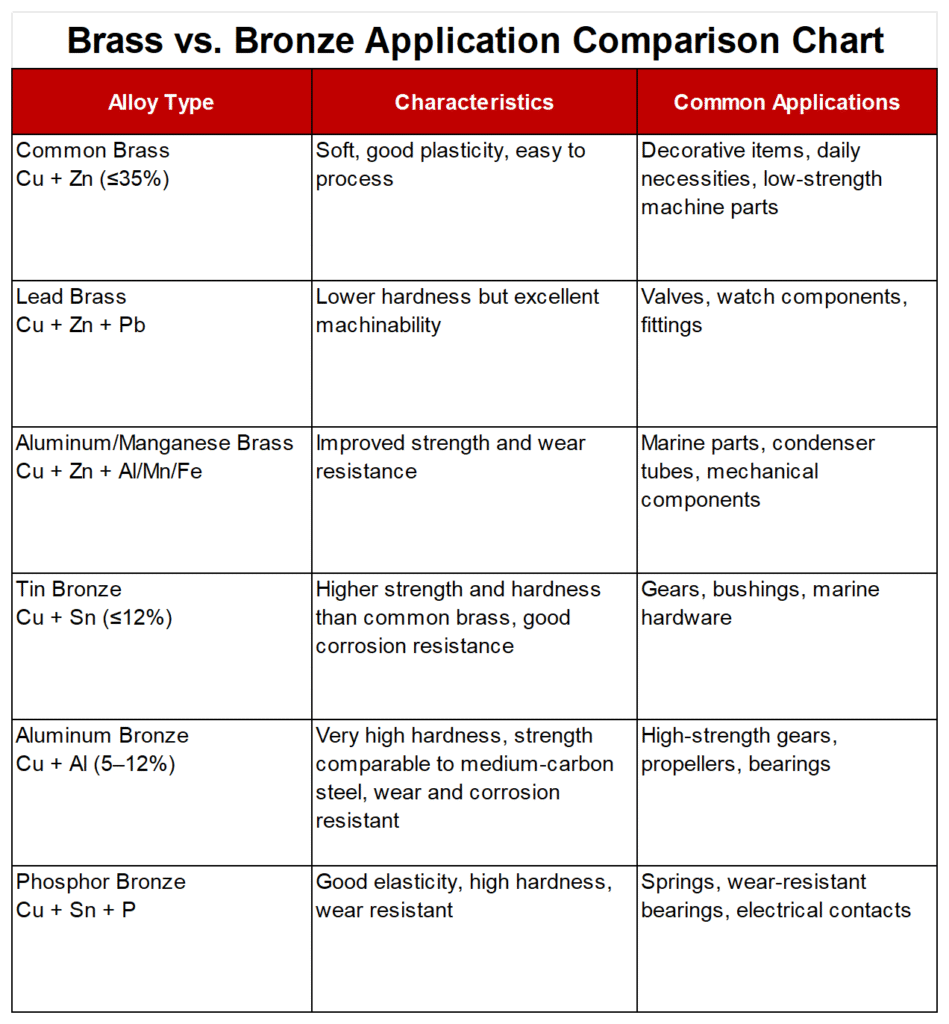
- Decoration & Home Hardware → Brass
- Musical Instrument Manufacturing → Brass
- Marine & Outdoor Applications → Bronze
- Bearings & Industrial Parts → Bronze
- Art Sculpture → Bronze
Based on the above comparison, I believe it’s easy to decide whether to choose brass or bronze. Indeed, we should choose one based on our needs and the application. Only by applying them in the most appropriate areas can we maximize their benefits. Below is a list of applications based on the characteristics of brass and bronze. We hope it can provide some general guidance if you’re still confused.
Impact of Supply and Demand on Prices
Generally speaking, bronze prices are typically higher than brass, primarily due to the relative scarcity and higher cost of tin. Both bronze and brass prices are directly impacted by the supply of raw materials (copper, zinc, and tin).
Take brass as an example: the global zinc market experienced a supply deficit and declining production in 2024, while the International Zinc Association (ILZSG) predicted a surplus of approximately 148,000 tons in 2025. What does this mean for you? Zinc prices could fall or stabilize, lowering brass production costs and making it easier for manufacturers to replenish raw materials, leading to increased production. For your processing industry, this could be a sourcing opportunity worth seizing.
However, I should also remind you that prices are not solely determined by supply. Continued increases in copper prices could offset some of the cost reductions brought about by zinc. Demand-side factors are also crucial: Growth in sectors like construction, electrical, and renewable energy, particularly strong demand from Asian markets such as China and India, is likely to continue to tighten raw material supply, thus supporting prices. Whether you are looking for cooperation in the processing industry or you are engaged in the processing industry yourself, as long as you pay attention to current affairs, many things may affect your profits.
Conclusion
Whether bronze or brass, each has its own unique advantages, which explains their widespread application in various fields. Understanding the differences between the two will help you confidently select the right material for your project, or find a partner who can match your needs. If you’re selecting metal for a project, consult with a professional supplier or fabricator; they can provide you with more practical advice and solutions.
FAQ
Is Brass Stronger than Bronze?
This question depends on the specific situation. Simply stating “brass is stronger than bronze” is inaccurate. Bronze is generally stronger, more wear-resistant, and more corrosion-resistant than brass. However, brass’s advantages lie in its workability and affordability, not simply in its perceived strength.
Can Brass or Bronze Be Used Outdoors?
Simply put, bronze is more reliable than brass because it can be placed outdoors in harsh environments or at the seaside. If it is just placed outdoors and is coated with a protective coating, brass can also be used.






